12+ titanium orbital diagram
This is called quantum jump. 121 Provide the orbital box diagram for titanium Ti.
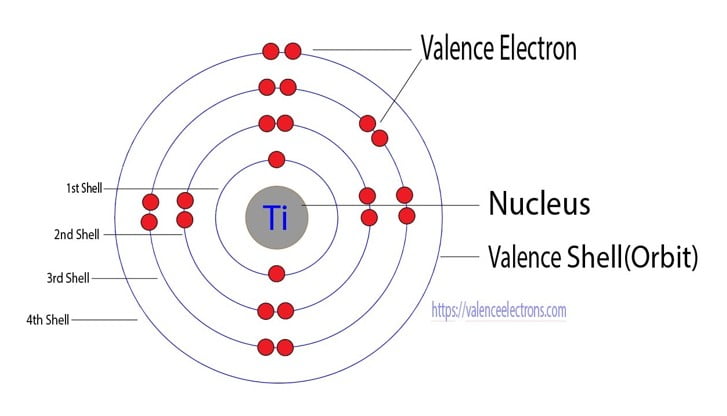
Titanium Ti Electron Configuration And Orbital Diagram
Apts Potentially useful information.

. Part A Choose the correct orbital diagram for titanium. Up to 256 cash back Get the detailed answer. If you are searching about in the ground-state electron configuration of fe3 how many unpaired youve visit to the right web.
To do that we need to find the number of e. For d subshell ℓ 2. Orbital diagram of Aluminum Al 14.
The orbital diagram will be filled in the same order as described by the Aufbau principle. Orbital diagram of Silicon Si 15. Orbital Notation orbital molecular diagram mo o2 li2 energy tungsten oxygen bef2 theory orbitals diagrams bond molecule valence chemistry wikipedia electron order titanium configuration.
Orbital diagram of Titanium Ti 23. 1 nm 10m. Located in the IV period.
Using an orbital box diagram and noble gas notation show the electron configuration of titanium. Of the four s and p orbitals are considered because these orbitals are the. The abbreviated ground electronic configuration for titanium is.
View the full answer. The order in which the orbitals are filled with electrons from lower energy to higher energy is. Orbital diagram of Magnesium Mg 13.
So each s subshell has one orbital each p subshell has three orbitals each d subshell has five orbitals and each f subshell has seven. So the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full. 451 gcm 3.
1s 2s. Orbital diagram for titaniumTi Titanium excited state electron configuration. Give one possible set of four.
T 1u a1g t2g eg a1g t1u eg a1g t1u a 1g e g eg t1u Δo t2g Metal Ti3orbitals Ti3 Ar 3d1 4s0 1e- Ligand group H2O orbitals 6 x 2 12 e- σ Ti H2O63. Here the energy of 4s orbital is less than that of 3d. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below.
Ar4s23d2 Electrons in atomic orbitals outside of the argon core Ar are the outermost electrons. For f subshell ℓ 3. Ar 1 11111 4s 3d Ar 111 4s 3d Ar 11 111 4s 3d.
Electronic configuration of the Titanium atom in. 1 nm 10m. Atoms can jump from one orbital to another orbital in an excited state.
We have 9 Pics about in the ground-state electron. To write the orbital diagram for the Titanium Ti first we need to write the electron configuration for just Ti. Ti Titanium is an element with position number 22 in the periodic table.
The orbital diagram for Titanium is drawn by following three principles the Aufbau principle Hunds principle and Paulis exclusion principle. The Titanium orbital diagram comprises. There are four different kinds of orbitals denoted s p d and f each with a different shape.
The method of entering electrons into. Apts Potentially useful information.
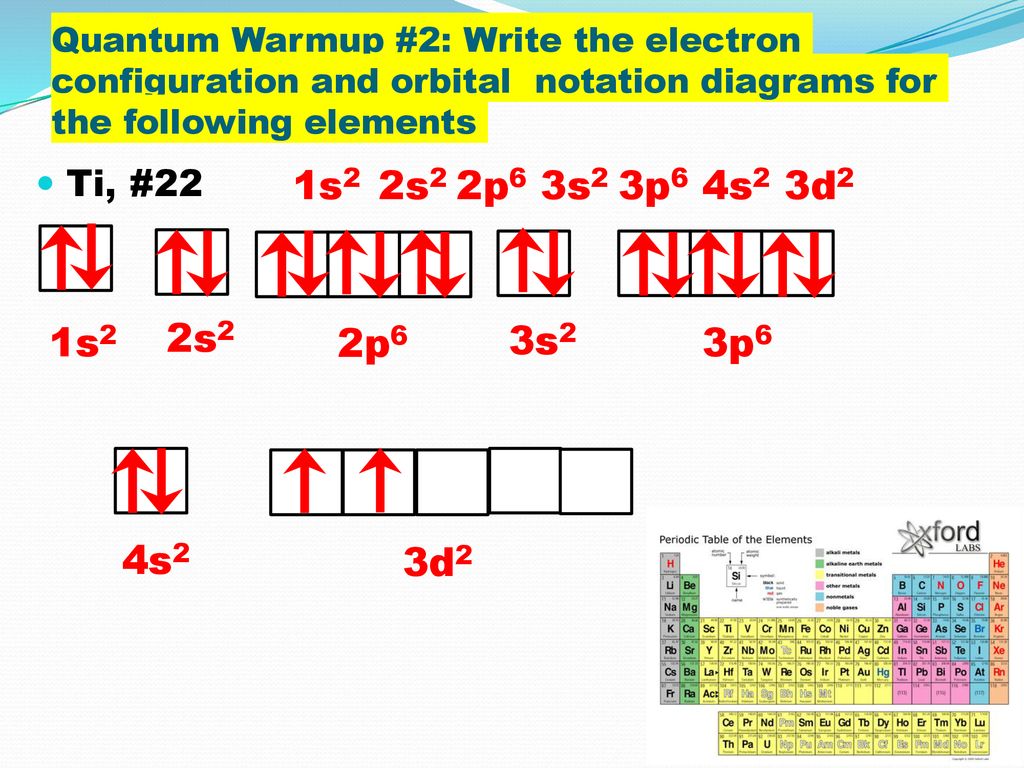
Electron Configurations And Orbital Notation Diagrams Ppt Download

Endohedrally Doped Cage Clusters Chemical Reviews
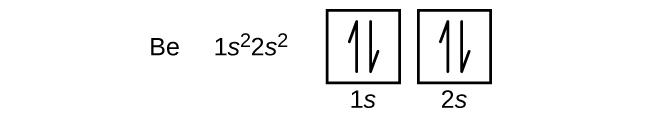
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts
Chem4kids Com Titanium Orbital And Bonding Info

Draw And Explain The Orbital Diagram For Titanium Homework Study Com
Chem4kids Com Titanium Orbital And Bonding Info

Titanium Electron Configuration Orbital Diagram Valence Electrons

Titanium Ti Electron Configuration And Orbital Diagram

Orbital Filling Diagrams The Cavalcade O Chemistry

How To Write The Atomic Orbital Diagram For Titanium Ti Youtube
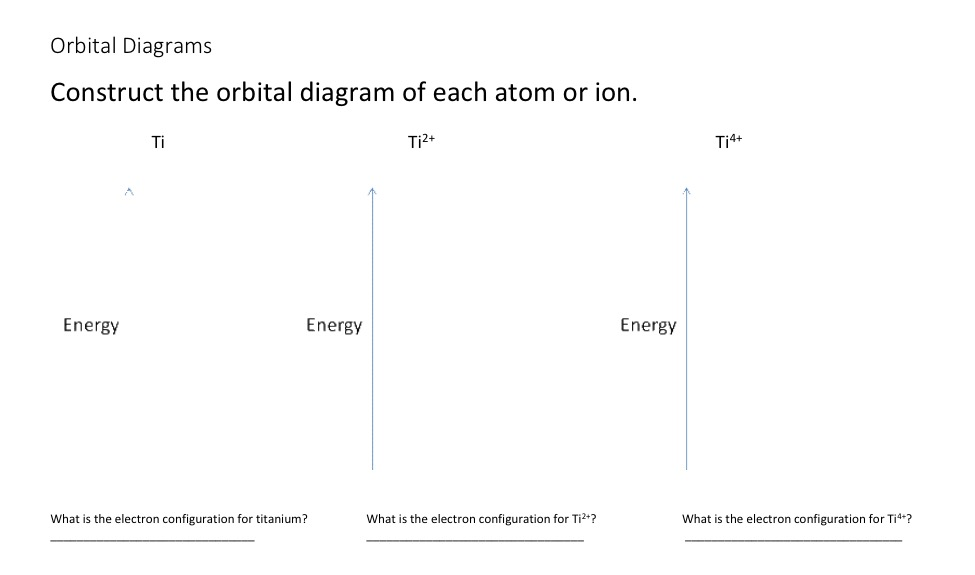
Solved Construct The Orbital Diagram Of Each Atom Or Ion Chegg Com
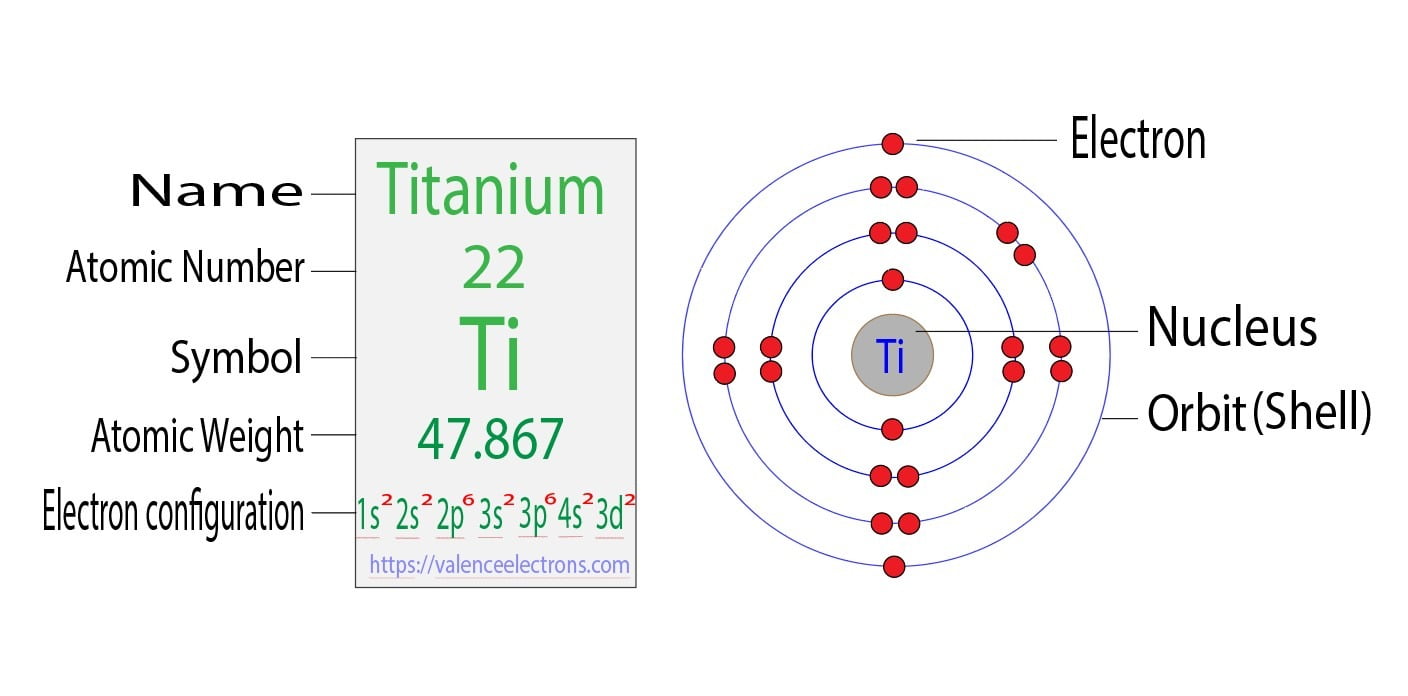
Titanium Ti Electron Configuration And Orbital Diagram

How To Write The Atomic Orbital Diagram For Titanium Ti Youtube

Orbital Filling Diagrams The Cavalcade O Chemistry
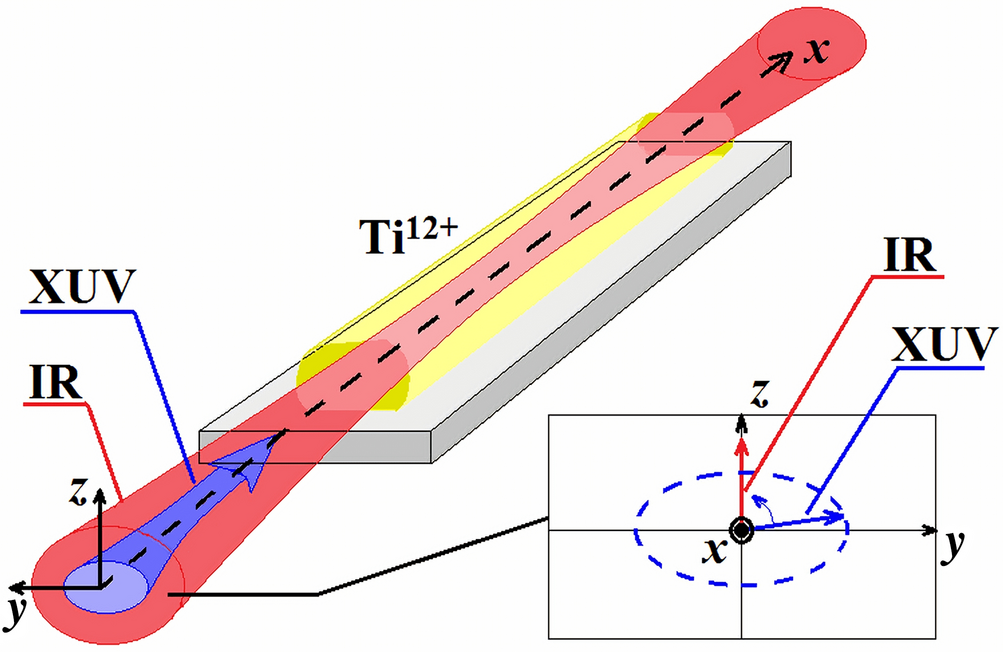
Amplification Of Elliptically Polarized Sub Femtosecond Pulses In Neon Like X Ray Laser Modulated By An Ir Field Scientific Reports

Titanium Electron Configuration Orbital Diagram Valence Electrons

Chemistry The Science In Context Volume I And Ii 4th Edition Gilbert